A compound is a substance formed when two or more chemical elements are chemically bonded together. In mixtures, the substances present are not chemically bonded together.

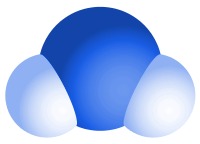
The type of bonds holding elements together in a compound can vary: two common types are covalent bonds and ionic bonds.
The elements in any compound are always present in fixed ratios.
Example 1: Pure water is a compound made from two elements - hydrogen and oxygen. The ratio of hydrogen to oxygen in water is always 2:1. Each molecule of water contains two hydrogen atoms bonded to a single oxygen atom.
Example 2: Pure table salt is a compound made from two elements - sodium and chlorine. The ratio of sodium ions to chloride ions in sodium chloride is always 1:1.
Example 3: Pure methane is a compound made from two elements - carbon and hydrogen. The ratio of hydrogen to carbon in methane is always 4:1.
Example 4: Pure glucose is a compound made from three elements - carbon, hydrogen, and oxygen. The ratio of hydrogen to carbon and oxygen in glucose is always 2:1:1.
Compounds can be decomposed chemically into their constituent elements.
Further differences between compounds and mixtures are listed in the definition of mixture.