Delocalization happens when electric charge is spread over more than one atom. For example, bonding electrons may be distributed among several atoms that are bonded together.

Example 1:
One of the best known examples of a molecule in which bonding electrons are delocalized is benzene, shown below:
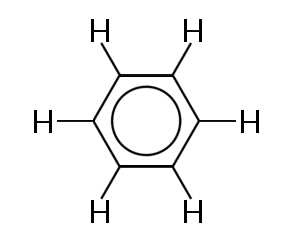
Benzene consists of a ring of six carbons atom. The carbon atoms are all sp2 hybridized with one unhybridized p orbital.
The sp2 hybrid orbitals produce normal covalent bonds, sometimes called σ-bonds: these are the single C-C bonds and single C-H bonds.
This leaves each carbon with an electron in a p orbital at a right angle to the plane of the ring.
In the diagram below, on the left you can see the sp2 orbitals forming covalent bonds. On the right, you can see the p orbitals, each of which contains an electron.
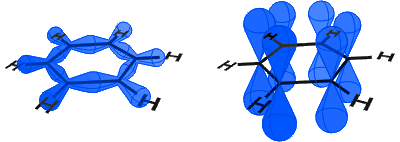
The p orbitals combine side on and the electrons in the p orbitals are described as π-electrons.
In the diagram below, the p orbitals have combined, and the π-electrons are delocalized. On average all of the ring bonds are identical.
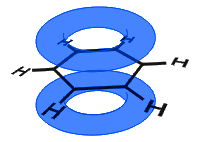
Unlike in the next the example below, the delocalized electrons in benzene are held within the molecule and are not free to move through the bulk material.
Example 2:
Metals have high thermal and electrical conductivity because the outermost electrons in their atoms are delocalized. These electrons are not associated with any particular metallic nucleus, and so are free to move throughout the metal.
Acknowledgement: The blue colored images of benzene's orbitals are courtesy of Sansculotte. These images are licensed under the Creative Commons Attribution-Share Alike 1.0 Generic license.