The pH scale is alternatively sometimes called the pH-acid-base scale and sometimes just the acid-base scale.

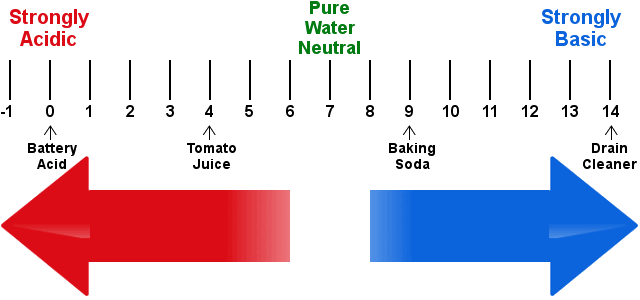
In neutral solutions, i.e. those that are neither acidic nor basic, pH = 7.0.
Acidic solutions are those with pH less than 7, while basic solutions have pH greater than 7.
A solution is:
- acidic when there are more H+ ions than OH- ions present
- basic when there are more OH- ions than H+ ions present
- neutral when there are equal number of H+ and OH- ions present
pH is a measure of the hydrogen ion activity, typically in aqueous solution.
In pH, the p stands for -log10 and the H stands for hydrogen ion activity:
In dilute solutions, the activity of hydrogen ions can be approximated by their molar concentration, and it is in this form that pH is normally used:
To see why this should be so, consider the following:
Water exists as an equilibrium between molecules and ions:
The multiplication product of the concentrations [H+] and [OH-] is a constant; it is water's dissociation constant, which has a known value of 10-14 M2.
For neutrality, [H+] must be equal to [OH-]. This means these quantities must both equal 10-7 M.
If we put [H+] = 10-7 M into the equation for pH:
we get pH = 7 for neutrality.
Acid solutions have pH < 7. pH values lower than 0 are possible.
Basic solutions have pH > 7. pH values greater than 14 are possible.