Solvation is the process by which solvent molecules surround and interact with solute ions or molecules.

An important specific example of solvation is hydration, where the solvent is water.
In general, the rule of like-attracts-like applies to solvation:
- Polar solutes such as sodium chloride are solvated by polar solvents such as water; they are not solvated by non-polar solvents such as benzene.
- Non-polar solutes such as icosane are solvated by non-polar solvents such as benzene; they are not solvated by polar solvents such as water.
- Soap molecules have a polar and non-polar part. The non-polar part attracts non-polar molecules such as grease, while the polar part allows solvation by water. This combination of properties means soapy water is an excellent mixture in which to, for example, wash dirty dishes after a meal.
A solvate is a compound that forms between a solute and its solvent.
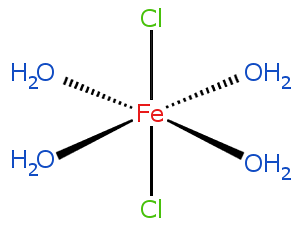
Hydrates:A wide range of salts form hydrates when dissolved in water. The liquid water can then be evaporated to leave the solid hydrate.
For example, ferrous chloride FeCl2 forms the hydrate FeCl2(H2O)4.
In addition to hydrates, other common solvates are ammoniates, alcoholates, and etherates.
The solvation number describes the number of solvent molecules bound to a solute species. For example, in FeCl2(H2O)4 above the solvation number is 4.