Absorption of light takes place when matter captures electromagnetic radiation, converting the energy of photons to internal energy. Energy is transferred from the radiation to the absorbing species.

Since the energy levels of matter are quantized, only light of energy that can cause transitions from one existing energy level to another will be absorbed.
We describe the energy change in the absorber as a transition or an excitation from a lower energy level to a higher energy level.
The amount of energy carried by a light photon depends on its wavelength. The shorter the wavelength, the higher the energy:
- ultraviolet or visible light absorption promotes electrons to higher orbitals or sublevels
- infrared light excites vibrations of molecules
- microwave wavelengths excite rotation of molecules
Absorption spectroscopy is one way to study the energy levels of atoms and molecules. An absorption spectrum measures the amount of light absorption as the light's wavelength is varied.
The spectrum of an atom or molecule depends on its energy-level structure. This makes absorption spectra useful for identifying elements and compounds, because each spectrum acts like a fingerprint.
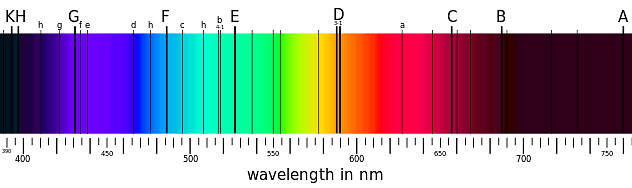
Absorption spectra are the means by which the chemical composition of our sun and other stars was discovered. The dark lines in the spectrum below correspond to elements present in the sun's atmosphere that absorb specific wavelengths of light.
Measuring the concentration of an absorbing species in a sample is accomplished by applying the Beer-Lambert Law.
