What does Amphiprotic mean?
An amphiprotic substance is one that can donate or accept H+ ions. The behavior generally depends upon the medium in which the substance is placed.

When an amphiprotic substance:
- donates H+, it is acting as an acid according to the Bronsted-Lowry definition of acid
- accepts H+, it is acting as a base according to the Bronsted-Lowry definition of base
The word amphiprotic comes from Greek, where Ampho means both or both kinds of behavior and protic refers to protons, in other words H+ ions.
Examples of Amphiprotic Behavior
In water, equilibrium exists between water molecules and ions produced by water's self-ionization. In the reaction equation below, you can see one water molecule donates H+ and another accepts H+. One molecule of water acts as a base, while another acts as an acid.
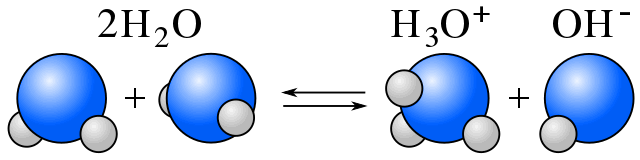
Water also shows amphiprotic behavior in the presence of other substances:
Example 1a: Water accepts H+, therefore acts as a base.
Example 1b: Water donates H+, therefore acts as an acid.
Amphiprotic vs Amphoteric
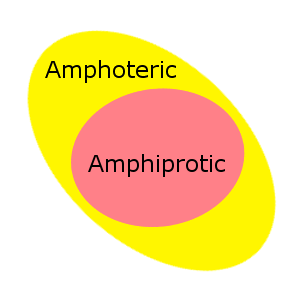
Amphiprotic is sometimes confused with amphoteric. While the words look similar, and have similar meanings, amphoteric is a broader term that encompasses Lewis acid-base behavior in addition to Bronsted-Lowry acid-base behavior.
All amphiprotic substances are also amphoteric.
Amphiprotic Substances
Water, hydrogen carbonate ions HCO3-, hydrogen sulfate ions HSO4-, and dihydrogen phosphate ions H2PO4- are common examples of amphiprotic species.
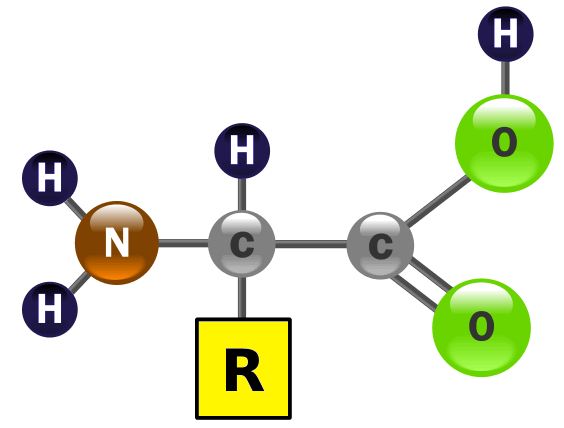
Amino acids contain acidic carboxyl groups (-COOH) and basic amine groups (-NH2) and are amphiprotic.