The Bronsted-Lowry definitions for acids and bases are:

Acid example:
In this example, HNO3 is an acid, donating a proton, and H2O is acting as a base, accepting a proton.
NO3- is called the conjugate base of the acid HNO3, and H3O+ is the conjugate acid of the base H2O.
Base example:
In this example, NH3 accepts a proton, acting as a base, and H2O is acting as an acid.
NH4+ is the conjugate acid of the base NH3, and OH- is the conjugate base of the acid H2O.
A compound that can act as either an acid or a base, such as the H2O in the above examples, is called amphoteric. Furthermore, H2O can be described as amphiprotic, because it can act as both a donor or acceptor of protons, H+.
The Bronsted-Lowry definition of acids and bases does not encompass all chemical compounds that exhibit acidic and basic properties. A more general definition is that of Lewis acids and bases:
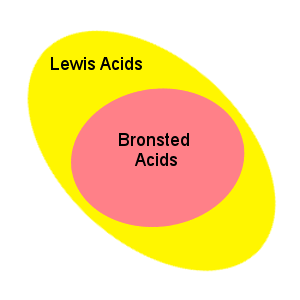
A Lewis acid is an electron pair acceptor; a Lewis base is an electron pair donor.
These definitions are broader than the Bronsted-Lowry definition in that they include many compounds that do not have protons, but exhibit acid/base behavior.
The Lewis definition encompasses the Bronsted-Lowry definition: In the reaction of H+ and OH-, H+ is a Lewis acid because it accepts an electron pair from the OH-. Since the OH- donates an electron pair we call it a Lewis base.
As an example of a reaction not described by the Bronsted-Lowry definition, Al3+ in water is a Lewis acid. It reacts with water to form an aqua complex: the Al3+ accepts an electron pair from water molecules with the water acting as a Lewis base.