A crystal is a solid composed of atoms, ions, or molecules arranged in a pattern that is periodic in three dimensions. [ASTM F1241]
The Unit Cell
Crystals are usually presented in terms of their unit cell. The unit cell is the smallest part of a crystal that, if repeated regularly by translation in three dimensions, creates the whole crystal. (Note: only translation of the unit cell is permitted, not inversion, rotation, or reflection.)
The unit cell of a crystal is defined by the crystal's lattice points.


Examples of Crystals
Nonmetal Elements
The carbon atoms in diamond are held together in a covalent lattice by network covalent bonding. Iodine crystals are made of I2 molecules held together in a crystal lattice by van der Waals forces. These forces are weak compared with covalent bonds, leading to a low melting point for iodine.


Metal Elements
Solid metal crystals are held together by metallic bonding: i.e. a lattice of positively charged metal ions is held together by sharing delocalized conduction electrons.


Metalloid Elements
Crystalline silicon is held together in a covalent lattice by network covalent bonding. Tellurium crystals are formed from spiral chains of covalently bonded atoms in a hexagonal lattice.

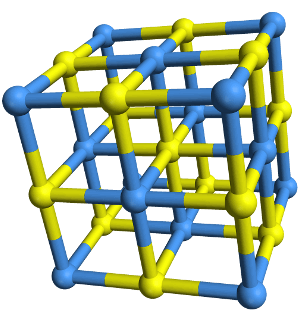
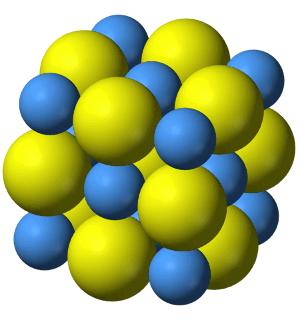
Ionic Compounds
Ionic solid compounds are held together in a lattice by ionic bonds between anions and cations.

Image by Crystal Titan.

Covalent Compounds 1
The covalent solid compounds below are held together in a covalent lattice by network covalent bonding.

Image by Didier Descouens.
Covalent Compounds 2
The water ice crystals below are held together in a lattice by polar covalent bonds.


Anisotropy
Differences in crystals when viewed along different axes can lead to anisotropy in crystalline materials.
Crystal Structure
The precise lattice constructions of crystals are found using X-ray diffraction.
Non-Crystalline Solids
Not all solids are crystalline. Solids also exist in amorphous and glass forms: unlike crystals, these forms have no long-range ordering in their structures.