What is an Ion?
An ion is an atom or molecule that carries an electric charge.
Ions are identified by the use of a superscript to show the sign and size of their electric charge.
Anions and Cations
Negatively charged ions are called anions, and positively charged ions are called cations.
Examples of Anions
- a bromine atom gains an electron to become Br-
- a sulfur atom gains two electrons to become S2-
- a nitrogen atom gains three electrons to become N3-
- phosphorus and oxygen form the molecular anion PO43-
Examples of Cations
- a sodium atom loses an electron to become Na+
- a magnesium atom loses two electrons to become Mg2+
- an iron atom loses three electrons to become Fe3+
- water reacts with H+ to form the molecular cation H3O+
Ion Formation
Ions form when doing so minimizes the total potential energy of the chemical species involved in the chemical reaction. Often, this is achieved by allowing atoms of different elements to achieve a full shell of electrons.
Consider the salt lithium fluoride:
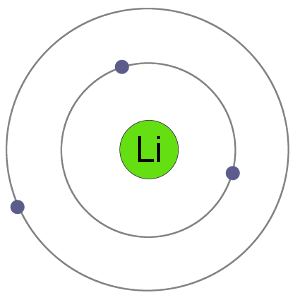
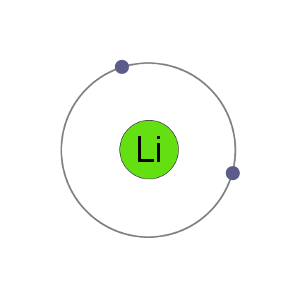
Lithium atoms have 3 electrons, meaning they have electrons in two shells: 2 in the first shell and 1 in the second. By losing an electron to become a cation, lithium gets a stable electron arrangement, identical to that of the noble gas helium.
Li Atom
Li+ Cation
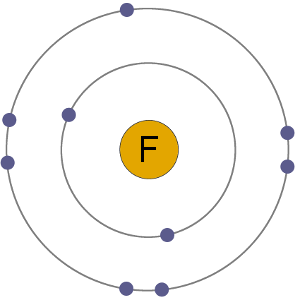
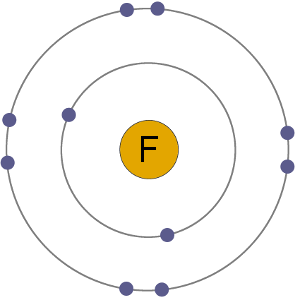
Fluorine atoms have 9 electrons, meaning they have 2 in the first shell and 7 in the second. By gaining an electron to become an anion the fluoride ion gets a stable electron arrangement, identical to that of the noble gas neon.
F Atom
F- Anion
And so the redox reaction of sodium with chlorine to form an ionic compound is energetically favorable:
Ion Sizes
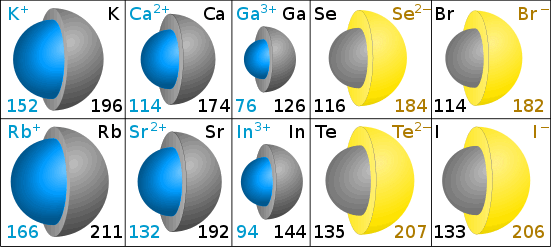
Cations are smaller than the original atom - consider how the sodium atom has electrons in three shells, while the sodium ion has electrons in only two shells. With more electrons, anions are larger than the original atom.
Ion sizes are measured by the ionic radius.
Ionic Radii in Picometers
The gray color in the diagrams shows the size of the original atom. The blue (cation) and yellow (anion) shows the size of the ion. (Diagrams by Chris King.)

Ionic Compounds
Ionic compounds, such as sodium chloride (NaCl) are formed because of the electrostatic attraction between anions and cations. Ionic compounds are electrically neutral, because the total number of positive charges and the total number of negative charges are always equal.
Considering the chemical elements, the highest tendency to form ionic compounds exists in the least electronegative metals and the most electronegative non-metals.
Electric Conductors
Ions can carry electric current; this can be observed in electrochemical cells, and in plasma, for example.
In general, solid ionic compounds do not carry electric current, because the ions are locked in fixed positions in a crystal lattice. Ionic compounds only conduct electricity when molten or when dissolved in solution, when the ions are released from the crystal lattice and become mobile.
References
The diagram of atomic radii was created by Popnose (Chris King) and is licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license.
