Electrochemistry is a sub-discipline of physical chemistry; it is concerned with:
- chemical reactions involving charged particles and electron transfers - i.e. redox reactions
- the relationship between electrical and chemical energy and the conversion of one to the other
Electrochemical processes are fundamental in a wide range of phenomena. For example, photosynthesis and brain chemistry are driven by electrochemical processes, as are electric batteries and fuel cells.
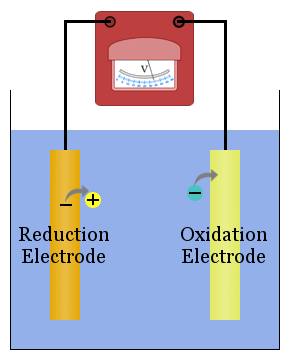
To study redox reactions, electrochemists usually find it convenient to separate the reduction and oxidation reactions in a cell using electrodes connected by a metal conductor to transport electrons from the species losing electrons to the species gaining electrons.
Equilibrium electrochemical methods allow redox potentials to be measured, from which crucial parameters such as the enthalpy, entropy, and Gibbs free energy can be determined for the reactions studied.
Chemical reaction rates can also be measured, because the reaction rate is related to the electric current - the higher the current, the faster the reaction. Techniques such as cyclic voltammetry and impedance spectroscopy may be used to determine reaction rates.
Electrochemistry is one of the main research and development disciplines involved in fields such as:
- fuel cells
- batteries
- metal corrosion protection
- electroplating
Instruments based on electrochemical methods are vital in analytical chemistry and provide convenient ways to measure, for example:
- pH
- water contamination
- toxic gas levels in air
- gene expression
- blood glucose
- alcohol in breath
