"Redox" is a word coined from two chemical terms:
• reduction = species gain electrons
•oxidation = species lose electrons
Redox reactions involve electron transfers; if one species gains electrons, another species (or more than one species) must lose electrons. For example, the redox reaction in which zinc displaces copper in an aqueous solution of copper sulfate:
In terms of electrons:
Reduction of Cu2+(aq)
and
Oxidation of Zn(s)
These spontaneous reactions (ΔG° for the reaction is -212.6 kJ mol-1) would take place directly between the species if zinc metal were added to a vessel containing copper sulfate solution, releasing heat.
In electrochemistry, however, these transfers take place at separate electrodes, releasing electrical energy, such as in the Daniell cell:
The Daniell Cell
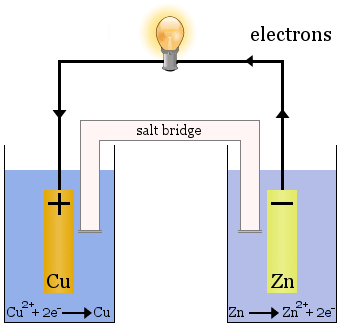
The Daniell cell is made up of two half-cells: copper ions are reduced in one and zinc is oxidized in the other.
When the cell operates, Cu2+ is removed from one cell and Zn2+ is produced in the other cell. The copper electrode begins to grow as it is plated with freshly deposited copper metal, and the zinc electrode begins to diminish due to the loss of zinc as ions into solution.
Energetically Uphill and Downhill
If the redox reaction is energetically downhill, as in the Daniell cell above, (i.e. spontaneous, with ΔG < 0) then the reaction can be used to power electric devices. Cells that power devices are known as galvanic cells.
If the redox reaction is energetically uphill (i.e. non-spontaneous, with ΔG > 0) then the reaction must be driven using an electric power source. This is usually done when one or more useful products are generated at separate electrodes. Cells that require an energy input are known as electrolytic cells.
