The chemical element argon is classed as noble gas and a nonmetal. It was discovered in 1895 by William Ramsay and Lord Rayleigh.

Data Zone
| Classification: | Argon is a noble gas and a nonmetal |
| Color: | colorless |
| Atomic weight: | 39.948 |
| State: | gas |
| Melting point: | -189.3 oC, 83.85 K |
| Boiling point: | -185.8 oC, 87.3 K |
| Electrons: | 18 |
| Protons: | 18 |
| Neutrons in most abundant isotope: | 22 |
| Electron shells: | 2,8,8 |
| Electron configuration: | 1s2 2s2 2p6 3s2 3p6 |
| Density @ 20oC: | 0.001784 g/cm3 |
Compounds, Radii, Conductivities
| Atomic volume: | 22.4 cm3/mol |
| Structure: | fcc: face-centered cubic when solid |
| Specific heat capacity | 0.520 J g-1 K-1 |
| Heat of fusion | 1.188 kJ mol-1 |
| Heat of atomization | 0 kJ mol-1 |
| Heat of vaporization | 6.447 kJ mol-1 |
| 1st ionization energy | 1520.5 kJ mol-1 |
| 2nd ionization energy | 2665.8 kJ mol-1 |
| 3rd ionization energy | 3930.8 kJ mol-1 |
| Electron affinity | – |
| Minimum oxidation number | 0 |
| Min. common oxidation no. | 0 |
| Maximum oxidation number | 0 |
| Max. common oxidation no. | 0 |
| Electronegativity (Pauling Scale) | – |
| Polarizability volume | 1.586 Å3 |
| Reaction with air | none |
| Reaction with 15 M HNO3 | none |
| Reaction with 6 M HCl | none |
| Reaction with 6 M NaOH | none |
| Oxide(s) | none |
| Hydride(s) | none |
| Chloride(s) | none |
| Atomic radius | 71 pm (measured) |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | – |
| Ionic radius (3+ ion) | – |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 1.77 x 10-2 W m-1 K-1 |
| Electrical conductivity | 0 mS cm-1 |
| Freezing/Melting point: | -189.3 oC, 83.85 K |

Solid argon at its melting point -189.3 oC. Image Ref (8).

The violet glow of ionized argon gas in a discharge tube. Image: Gianfuffo.
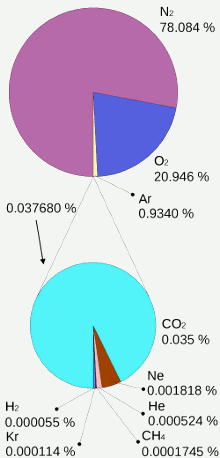
The percentage volume of each gas in earth’s dry atmosphere. In practice water vapor is also present. Image: Mysid.
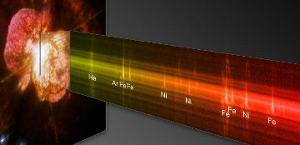
A faint line from argon shows in the spectrum of the doomed star Eta Carinae. Eta Carinae has a mass of more than 100 Earth suns. William Ramsay discovered argon when he first saw its spectrum and realized it matched no other’s. Image: NASA, ESA, and the Hubble SM4 ERO Team.
Discovery of Argon
Argon was the first noble gas to be discovered.
The first hint of its existence came from English scientist Sir Henry Cavendish as far back as 1785. Cavendish was unhappy that so little was known about air. He was particularly unhappy about the lack of information about the fraction of air (the majority) which was not oxygen. (1)
He knew the nitrogen in air could be reacted with oxygen to form, ultimately, nitrous acid. He aimed to find out if ALL of the air that was not oxygen or carbon dioxide could be converted to nitrous acid. If it could, he would know that air was entirely oxygen, carbon dioxide and nitrogen.
Cavendish used an electric spark in air to react the oxygen and nitrogen to form nitrogen oxides. He then added additional oxygen until all the nitrogen had reacted.
Nitrogen oxides are acidic. Cavendish used aqueous sodium hydroxide to remove them from the apparatus. [This would also, of course, have removed any carbon dioxide that was present.] He removed the remaining oxygen using potassium polysulfides.
A small bubble of gas remained [mostly argon]. Cavendish wrote that this bubble “was not more than one hundred and twentieth of the bulk of the phlostigated air [nitrogen].” (1) So, Cavendish is saying that air is at least 99.3 percent nitrogen/oxygen/carbon dioxide with a maximum 0.7 percent of something else. We now know that the ‘something else’, argon, is very unreactive; this enabled Cavendish to find it, but it also prevented him finding out more about it. (The giant advances in spectroscopy made by Gustav Kirchhoff and Robert Bunsen lay 85 years in the future.)
In hindsight, we can say Cavendish slightly underestimated the part of air that isn’t oxygen, nitrogen, or carbon dioxide. Despite this, he was ahead of his time. After his experiment, more than 100 years passed until scientists again began to think that something about air didn’t quite add up.
In 1892 English physicist John William Strutt (better known as Lord Rayleigh) announced that no matter how it was prepared, oxygen was always 15.882 times denser than hydrogen. This very precise work had taken ten years to complete.
Continuing to work with great attention to detail, he found that the ‘nitrogen’ in air was always denser by about 0.5 percent than nitrogen sourced from nitrogen compounds. (2), (3) How could this be explained? In 1893 he wrote to Nature, announcing the problem to the world. Any scientist who responded to that challenge actually had the chance of discovering a new element. None did!
In April 1894 Rayleigh wrote an academic paper about the nitrogen problem. Funnily enough, Rayleigh viewed pure nitrogen, containing no argon, as ‘abnormally light nitrogen.’ He stored it for eight months and retested it to see whether its density would increase. (4)
Rayleigh’s paper awakened the serious interest of Scottish chemist William Ramsay, who had already been aware of the problem.
Rayleigh and Ramsay carried out further experiments, keeping in touch with one another about their progress.
In August 1894 Ramsay took air and removed its components – oxygen, carbon dioxide and nitrogen. He removed the nitrogen by reacting it with magnesium. After removing all the known gases from air, he found gas remaining that occupied one-eightieth of the original volume. Its spectrum matched no known gas.
Rayleigh and Ramsay wrote a joint paper in 1895 notifying the world of their discovery. The new gas wouldn’t react with anything, so they named it argon, from the Greek ‘argos’, meaning inactive or lazy. (5)
In his Nobel Prize winning address, Rayleigh said: “Argon must not be deemed rare. A large hall may easily contain a greater weight of it than a man can carry.” (6) William Ramsay discovered or codiscovered most of the other noble gases: helium, neon, krypton and xenon.
He was responsible for adding an entire new group to the periodic table. Radon was the only noble gas he didn’t discover.
Interesting Facts about Argon
- Lord Rayleigh said: “Argon must not be deemed rare. A large hall may easily contain a greater weight of it than a man can carry.” On a planetary scale, we can calculate that Earth’s atmosphere holds 65 trillion metric tons of argon. That’s more than 9 metric tons of argon per person on Earth.
- Until 1957, argon’s chemical symbol was A. In 1957, IUPAC agreed that the symbol should change to Ar. Argon was not the only element whose symbol changed in 1957. IUPAC also changed mendelevium from Mv to Md.
- Most people are familiar with carbon dating, which uses the decay of the radioactive carbon-14 isotope to find the ages of things that were once alive. Carbon-14’s half-life is about 5730 years and the technique is not useful for material more than about 60 thousand years old. Potassium-argon and argon-argon dating allow us to date rocks that are much older than this. Potassium-40 decays to argon-40 and calcium-40, with a half-life of 1.25 billion years. The ratio of potassium-40 to argon-40 trapped in rock can be used to determine how long it is since the rock has solidified. More recently, the ratio of argon-39 to argon-40 has been used in precision dating.
- The vast majority of argon on Earth comes from the radioactive decay of potassium-40, producing stable argon-40. Over 99% of Earth’s argon is argon-40.
- Away from Earth, argon-36 is the most abundant isotope, synthesized in the silicon burning phase of stars with a mass of about 11 or more Earth suns. During silicon burning, an alpha-particle adds to a silicon-32 nucleus to make sulfur-36, which can add another alpha-particle to become argon-36, some of which can become calcium-40, etc.


Appearance and Characteristics
Harmful effects:
Argon is considered to be non-toxic.
Characteristics:
Argon is a noble gas. It is colorless, odorless and extremely unreactive.
It is, however, not completely inert – photolysis of hydrogen fluoride in a solid argon matrix at 7.5 kelvin yields argon fluorohydride, HArF.
Argon forms no stable compounds at room temperature.
Uses of Argon
As a result of its unreactiveness, argon is used in light bulbs to protect the filament and to provide an unreactive atmosphere in the vicinity of welding.
It is also used in the semi-conductor industry to provide an inert atmosphere for silicon and germanium crystal growth.
Argon is used in medical lasers, in ophthalmology for example to correct eye defects such as blood vessel leakage, retinal detachment, glaucoma and macular degeneration.
Argon has low thermal conductivity and is used as the gas between the glass panes in high-efficiency double and triple glazing.
Abundance and Isotopes
Abundance earth’s crust: 3.5 parts per million by weight, 1.8 parts per million by moles
Abundance solar system: 0.01 percent by weight, 3.3 parts per million by moles
Cost, pure: $0.5 per 100g
Cost, bulk: $ per 100g
Source: Argon is produced when 40K present naturally in the earth’s crust undergoes radioactive decay to 40Ar. The argon makes its way into the atmosphere. Argon is produced commercially by fractional distillation of liquefied air with (for high purity argon) catalytic burning of left over traces of oxygen.
Isotopes: 18 whose half-lives are known, mass numbers 30 to 47. Of these, three are stable. They are found naturally in the percentages shown: 36Ar (0.337%), 38Ar (0.063%) and 40Ar (99.600%).

References
- Encyclopaedia Perthensis, or, Universal dictionary of the arts, Sciences, Literature, &c., 1816, vol 1, p231-232, John Brown.
- John H. Wolfenden, The Noble Gases and the Periodic Table: Telling it like it was., J. Chem. Educ., 1969, 46 (9), p569.
- Mary Elvira Weeks, The Discovery of the Elements. XVIII. The Inert Gases., J. Chem. Educ., 1932, 9 (12), p2065.
- Lord Rayleigh, On an Anomaly Encountered in Determinations of the Density of Nitrogen Gas., Proc. Roy. Soc. London, 1894, 55, p340.
- Vivi Ringnes, Origin of the Names of Chemical Elements, J. Chem. Educ., 1989, 66 (9), p731.
- Lord Rayleigh, The Density of Gases in the Air and the Discovery of Argon, Nobel Lecture, December 12, 1904. (pdf download.)
- Robert L. Kelly, David Hurst Thomas, Archaeology., Sixth Edition, 2012, Wadsworth, p137.
- Image by Deglr6328.
Cite this Page
For online linking, please copy and paste one of the following:
<a href="https://www.chemicool.com/elements/argon.html">Argon</a>
or
<a href="https://www.chemicool.com/elements/argon.html">Argon Element Facts</a>
To cite this page in an academic document, please use the following MLA compliant citation:
"Argon." Chemicool Periodic Table. Chemicool.com. 15 Oct. 2012. Web. <https://www.chemicool.com/elements/argon.html>.
fascinating and very thorough
This is the best site I’ve ever been to for Science
This site was very helpful and really made my chemistry project easy. Thank you.
that caveman picture is wierd
This is the best site I’ve ever been to for Elements info .
Great Job
Thanks Dr. Doug Stewart
this was very helpful especially towards my project….
Thanks!!!!!!
it would be perfect if it included chemical and physical properties of the element.
Thanks Bella, you can find the properties in the Data Zone by scrolling to the top of the page. Some of the properties, such as the reactions, are chemical properties, and others, such as electrical conductivity and melting point, are physical.
This website has helped me with my science homework thanks
this website helped me with my science project…. THANKS XD
this is also helping me too lol… I wanted potassium at first but this is better lol
is argon brittle or malleable and is it ductile or not ductile
Hi Anya, thanks for your question. Under normal conditions argon is a gas. Brittle/malleable/ductile are properties of solids, so they don’t really apply to argon.