The chemical element hafnium is classed as a transition metal . It was discovered in 1923 by Georg von Hevesy and Dirk Coster.

Data Zone
| Classification: | Hafnium is a transition metal |
| Color: | silvery |
| Atomic weight: | 178.49 |
| State: | solid |
| Melting point: | 2230 oC, 2503 K |
| Boiling point: | 4600 oC, 4873 K |
| Electrons: | 72 |
| Protons: | 72 |
| Neutrons in most abundant isotope: | 108 |
| Electron shells: | 2,8,18,32,10,2 |
| Electron configuration: | [Xe] 4f14 5d2 6s2 |
| Density @ 20oC: | 13.2 g/cm3 |
Reactions, Compounds, Radii, Conductivities
| Atomic volume: | 13.6 cm3/mol |
| Structure: | hcp: hexagonal close pkd |
| Hardness: | 5.5 mohs |
| Specific heat capacity | 0.14 J g-1 K-1 |
| Heat of fusion | 27.2 kJ mol-1 |
| Heat of atomization | 621 kJ mol-1 |
| Heat of vaporization | 575.0 kJ mol-1 |
| 1st ionization energy | 658.5 kJ mol-1 |
| 2nd ionization energy | 1440 kJ mol-1 |
| 3rd ionization energy | 2250 kJ mol-1 |
| Electron affinity | 178 kJ mol-1 |
| Minimum oxidation number | 0 |
| Min. common oxidation no. | 0 |
| Maximum oxidation number | 4 |
| Max. common oxidation no. | 4 |
| Electronegativity (Pauling Scale) | 1.3 |
| Polarizability volume | 16.2 Å3 |
| Reaction with air | mild, w/ht ⇒ HfO2 |
| Reaction with 15 M HNO3 | passivated |
| Reaction with 6 M HCl | none |
| Reaction with 6 M NaOH | none |
| Oxide(s) | HfO2 |
| Hydride(s) | HfH2 |
| Chloride(s) | HfCl4 |
| Atomic radius | 155 pm |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | – |
| Ionic radius (3+ ion) | – |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 23.2 W m-1 K-1 |
| Electrical conductivity | 3.4 x 106 S m-1 |
| Freezing/Melting point: | 2230 oC, 2503 K |

Hafnium metal is a silver color. It normally appears rather gray because of the oxide layer it forms in air. Hafnium powder, without the oxide layer, can ignite spontaneously in air. Image Ref. (1)
Discovery of Hafnium
Hafnium’s existence was originally predicted by Russian chemist Dmitri Mendeleev. In his 1869 book “The Periodic Law of the Chemical Elements” he predicted the existence of an element with similar properties to, but heavier than titanium and zirconium.
In 1911, Georges Urbain, the discoverer of the rare earth lutetium, thought he had discovered element 72 during his spectral analysis of rare earths. He called this new element celtium, but three years later it was shown to be a mixture of already discovered lanthanides. (2)
In 1921, Neils Bohr suggested to Hungarian chemist Georg von Hevesy to look for the missing element in zirconium ores. (3) According to Bohr’s quantum theory of atomic structure, these metals would have similar chemical properties, so there was a good chance they would be found in the same ores.
Following Bohr’s advice, Hevesy and Dutch physicist Dirk Coster discovered hafnium in 1923 using x-ray spectroscopy to analyze zirconium ores.
Anton Eduard van Arkel and Jan Hendrik de Boer discovered a method for producing high purity hafnium in 1925. Hafnium tetraiodide (HfI4) is decomposed on a white hot tungsten filament creating a crystal bar of pure hafnium. This is known as the crystal bar process.
The element was called Hafnium after the Latin name ‘Hafnia,’ meaning Copenhagen, the city where the element was discovered.

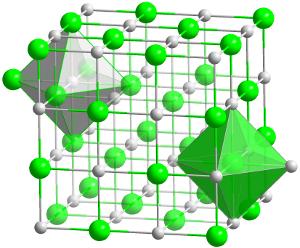
Crystal structure of hafnium carbide (HfC). This is the most refractory (heat resistant) compound known of any two elements in 1:1 ratio.

Hafnium is used in nuclear reactor control rods in nuclear submarines

White hafnium (IV) oxide (HfO2). Hafnium oxide based compounds are being introduced into silicon chips to produce smaller, high performance processors with improved energy efficiency.
Appearance and Characteristics
Harmful effects:
Hafnium is considered to be non-toxic. In powdered form it is pyrophoric (can ignite spontaneously).
Characteristics:
Hafnium is a lustrous, silvery, ductile metal.
Chemically it is similar to zirconium.
When present in compounds, hafnium exists mostly in the oxidation state IV.
Hafnium resists corrosion due to the formation of an oxide film on exposed surfaces.
It is unaffected by all acids (apart from hydrogen fluoride) and all alkalis.
Hafnium reacts with the halogens to form tetrahalides, and at high temperatures it reacts with carbon, boron, nitrogen, oxygen, silicon and sulfur.
Uses of Hafnium
Hafnium is used for nuclear reactor control rods because of its ability to absorb neutrons and its good mechanical and corrosion resistance qualities. This is in complete contrast to zirconium, which although is chemically is very similar to hafnium, is very poor at absorbing neutrons. Zirconium is therefore used in the cladding (outer layer) of fuel rods through which it is important that neutrons can travel easily.
Hafnium is also used in photographic flash bulbs, light bulb filaments, and in electronic equipment as cathodes and capacitors.
Hafnium alloys with several other metals, such as iron, niobium, tantalum and titanium.
Hafnium-niobium alloys, for example, are heat resistant and are used in aerospace applications, such as space rocket engines.
Hafnium carbide is used to line high temperature furnaces / kilns due to its refractory properties (it does not melt at high temperatures).
Hafnium-based compounds are used in gate insulators in the 45 nm generation of integrated circuits for computers.
Hafnium oxide-based compounds are being introduced into silicon-based chips to produce smaller, more energy efficient and performance packed processors(4).
Abundance and Isotopes
Abundance earth’s crust: 3.3 parts per million by weight, 0.4 parts per million by moles
Abundance solar system: 1 part per billion by weight, 0.01 parts per billion by moles
Cost, pure: $120 per 100g
Cost, bulk: $ per 100g
Source: Hafnium is not found free in nature but is found in most zirconium minerals at a concentration of between one and five percent. Commercially, hafnium is produced as a by-product of zirconium refining. This is done using the Kroll Process, reducing the tetrachloride with magnesium or with sodium.
Isotopes: Hafnium has 32 isotopes whose half-lives are known, with mass numbers 154 to 185. Naturally occurring hafnium is a mixture of six isotopes and they are found in the percentages shown: 174Hf (0.2%), 176Hf (5.3%), 177Hf (18.6%), 178Hf (27.3%), 179Hf (13.6%) and 180Hf (35.1%). The most abundant is 180Hf at 35.1%.

References
- Photo by Deglr6328
- Per Enghag, Encyclopedia of the Elements: Technical Data – History – Processing – Applications, 2008, John Wiley & Sons, p527.
- Bob Weintraub, George De Hevesy (1885 1966) (pdf document).
- Aile Tamm, Atomic Layer Deposition Of High-Permittivity Insulators From Cyclopentadienyl-Based Precursors, 2010, Tartu University Press, p18.
Cite this Page
For online linking, please copy and paste one of the following:
<a href="https://www.chemicool.com/elements/hafnium.html">Hafnium</a>
or
<a href="https://www.chemicool.com/elements/hafnium.html">Hafnium Element Facts</a>
To cite this page in an academic document, please use the following MLA compliant citation:
"Hafnium." Chemicool Periodic Table. Chemicool.com. 17 Oct. 2012. Web. <https://www.chemicool.com/elements/hafnium.html>.