What is Ionic Bonding?
Chemical bonds form when the total energy of the bonded atoms is lower than the total energy of the separate atoms. The form the bonding takes is determined by the electron arrangement that minimizes the energy.
In some instances, electrons are shared between atoms - this is termed covalent bonding.
In other instances, there is a complete transfer of one or more electrons from one atom to another. The atom that loses electrons becomes a positively charged ion. The atom that receives electrons becomes a negatively charged ion.
This leads to ionic bonding - the mutual electrostatic attraction of positive and negative charges. In its purest form, ionic bonding is not directional. It can be regarded as simple Coulombic attraction between point charges. This is different from covalent bonding, in which the sharing of electrons results in directional bonds.
Electron Transfer
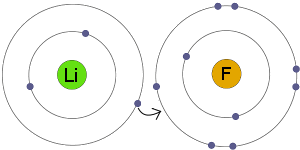
Li transfers an electron to F. The result is that both the resulting ions become electrically charged and have complete, stable electron shells.
Electrostatic Attraction
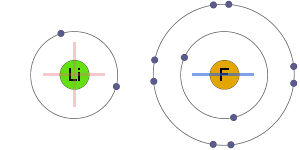
The positive and negative ions are electrostatically attracted to one-another, resulting in an ionic bond.
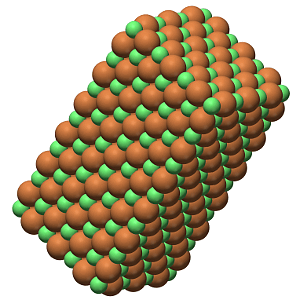
The coulombic force has no preferred direction, with the result that ionic compounds tend to exist as giant crystal lattice structures of ions packed together.
A Sample of Lithium Fluoride's Crystal Lattice
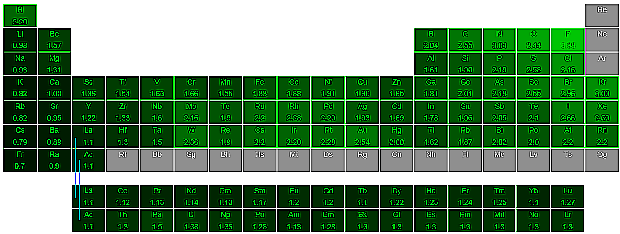
Electronegativity and Ionic Bonding
Two elements will tend to bond ionically when they differ widely in electronegativity.
Periodic Table of Electronegativity
The lighter the shade of green, the higher the electronegativity. Gray means no value is known. (Click image for larger view.)
Covalent Bonding Character
Absolutely pure ionically bonded compounds do not truly exist, because the positively charged ion polarizes the negative ion's electron cloud. This is particularly noticeable when dealing with small, highly charged metal ions such as Al3+, although the polarizing effect is present to some extent in all ionic compounds. Polarization is effectively a directional, electron sharing effect, hence it introduces a small amount of covalency in ionic compounds.
The proportion of ionic/covalent behavior in a bond can be very roughly assessed using the Pauling scale of electronegativity. Linus Pauling estimated that an electronegativity difference of 1.7 between elements leads to bonding that is 50 percent ionic.
Values below 1 correspond to covalent bonding dominating and values above 2 to ionic bonding dominating.
Electronegativity of Selected Elements (Pauling Scale)
| Element | Electronegativity |
|---|---|
| hydrogen | 2.20 |
| lithium | 0.98 |
| sodium | 0.93 |
| aluminum | 1.61 |
| carbon | 2.55 |
| oxygen | 3.44 |
| chlorine | 3.16 |
| iodine | 2.66 |
The C-H bond with a difference of 0.35 will be covalent, while NaCl bonding at 2.23 will be ionic. HCl bonding at 0.96 will be polar covalent, and LiI bonding at 1.68 will be 50/50 ionic/covalent.
The largest electronegativity difference is 3.19, between cesium (0.79) and fluorine (3.98). The resulting bonding is about 95 percent ionic in character.
