What is a Plasma?
Plasmas are one of the four common states of matter - the others are solids, liquids, and gases.
In general, but not in all specific cases, as the temperature increases and pressure decreases substances pass through the four different states.
solid → liquid → gas → plasma
Almost all the observable matter in the universe exists in the plasma state.
The Plasma State
The plasma state of matter is characterized by the following:
- charge separation by ionization: a plasma is a gas whose atoms have lost some or all of their electrons - it is a gas of ions and electrons
- a plasma has no definite shape or volume
- plasmas are overall electrically neutral, containing balanced numbers of positive and negative charges.
- plasmas are electric conductors, whereas gases are insulators
- plasmas form at very high temperatures: the higher the temperature and the lighter the element, the more fully ionized the plasma's atoms are likely to be:
- the solar corona and the interior of the sun are fully ionized plasmas
- lightning strikes consist of partially ionized plasmas, as do electric sparks
- lights based on ionized noble gases, such as neon lights, contain partially ionized plasma
- at lower temperatures, plasma may contain un-ionized neutral species, whose proportion falls as the temperature rises
- average speeds of particles in plasmas are higher than in gases, with a higher proportion of atomic- and molecular-sized particles moving at very high speeds
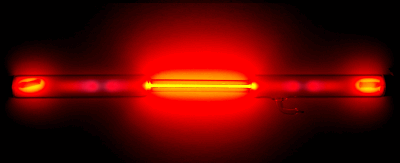
The image below is a neon light. A high voltage is applied to neon at low pressure, ionizing it to produce a plasma.