The chemical element yttrium is classed as a transition metal and rare earth metal. It was discovered in 1794 by Johan Gadolin.

Data Zone
| Classification: | Yttrium is a transition metal & rare earth |
| Color: | silvery-white |
| Atomic weight: | 88.9059 |
| State: | solid |
| Melting point: | 1525 oC, 1798 K |
| Boiling point: | 3340 oC, 3613 K |
| Electrons: | 39 |
| Protons: | 39 |
| Neutrons in most abundant isotope: | 50 |
| Electron shells: | 2,8,18,9,2 |
| Electron configuration: | [Kr] 4d1 5s2 |
| Density @ 20oC: | 4.47 g/cm3 |
Reactions, Compounds, Radii, Conductivities
| Atomic volume: | 19.8 cm3/mol |
| Structure: | hcp: hexagonal close packed |
| Specific heat capacity | 0.30 J g-1 K-1 |
| Heat of fusion | 11.40 kJ mol-1 |
| Heat of atomization | 423 kJ mol-1 |
| Heat of vaporization | 363.0 kJ mol-1 |
| 1st ionization energy | 615.6 kJ mol-1 |
| 2nd ionization energy | 1181 kJ mol-1 |
| 3rd ionization energy | 1979.9 kJ mol-1 |
| Electron affinity | 29.6 kJ mol-1 |
| Minimum oxidation number | 0 |
| Min. common oxidation no. | 0 |
| Maximum oxidation number | 3 |
| Max. common oxidation no. | 3 |
| Electronegativity (Pauling Scale) | 1.22 |
| Polarizability volume | 22.7 Å3 |
| Reaction with air | vigorous, ⇒ Y2O3 |
| Reaction with 15 M HNO3 | vigorous, ⇒ Y(NO3)3 |
| Reaction with 6 M HCl | mild, ⇒ H2, YCl3 |
| Reaction with 6 M NaOH | none |
| Oxide(s) | Y2O3 |
| Hydride(s) | YH2, YH3 |
| Chloride(s) | YCl3 |
| Atomic radius | 180 pm |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | – |
| Ionic radius (3+ ion) | 104 pm |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 17.2 W m-1 K-1 |
| Electrical conductivity | 1.8 x 106 S m-1 |
| Freezing/Melting point: | 1525 oC, 1798 K |

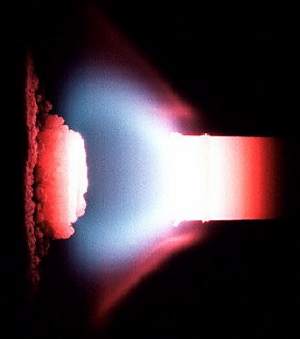
Rocket combustion chamber. The silver-colored lining is an alloy of nickel,chromium, aluminum and yttrium. Photo: NASA.

Yttrium is used in many applications, such as cubic zirconia gems, computer monitors, camera lenses and energy-efficient lighting.
Discovery of Yttrium
The story of yttrium’s discovery begins in 1787, when Carl Arrhenius found a coal-like mineral in a feldspar/quartz mine near Ytterby, Sweden. The mine had been developed in the early 18th century as a result of the mineral requirements of the local pottery industry.
Arrhenius called the black mineral ytterbite after Ytterby. Bengt Geijer, the inspector of mines in Stockholm, carried out a rough analysis of ytterbite. He reported that the mineral contained iron and speculated that it might also contain tungsten. (1), (2)
Johan Gadolin received a ytterbite sample from Arrhenius and carried out a detailed analysis of it in 1794, in Finland. He found it contained 31% silica, 19% alumina, 12% iron oxide and 38% of an unknown earth. (3)
Gadolin’s results were confirmed in 1797 by Swedish chemist Anders Ekeberg. Ekeberg suggested the name yttria for the oxide of the new earth metal and hence the new metal was named yttrium. (2)
Unfortunately Gadolin and Ekeberg did not realize that their alumina analyses were incorrect. The substance they identified as alumina was actually the oxide of another new element, beryllium.
Beryllium was discovered a year later, in 1798, by French chemist Nicolas Louis Vauquelin. Ekeberg then confirmed beryllium oxide was present in ytterbite and alumina was absent. (2)
Ytterbite was renamed gadolinite (a yttrium-iron-beryllium-silicate mineral) in 1800 by Martin Klaproth in honor of John Gadolin.
Gadolin tested the properties of yttria (yttrium oxide) in detail and found it did not melt even at the highest temperatures of the blowpipe; it also formed a clear colorless glass with borax. (3) (These were to be typical properties of all the rare earth metal oxides.)
Yttrium was the first rare earth element to be discovered. We now know Gadolin’s yttria was impure; in addition to yttrium oxide, it contained eight other rare earth metal oxides. These were discovered separately in later years; these metals were: erbium, terbium, ytterbium, scandium, thulium, holmium dysprosium, and lutetium.
Yttrium metal was first obtained in 1828, in Berlin, by Friedrich Wöhler as a gray powder by heating anhydrous yttrium (III) chloride with potassium. (4)
The metal was produced with high purity in 1953 by Frank Spedding at Ames Laboratory, in Iowa, using ion exchange techniques. (5)


Ultra-pure yttrium-90 is used for cancer therapy. Yttrium-90 is produced by high-purity separation from strontium-90, a fission product of uranium in nuclear reactors. Photo: PNNL
Carbon nanotubes are produced from carbon vapor containing a small amount of nickel and yttrium catalysts. An electric arc vaporizes an anode containing the catalysts. Photo: NASA.
Appearance and Characteristics
Harmful effects:
Water soluble compounds of yttrium are considered to be slightly toxic, while its insoluble compounds are considered to be non-toxic.
Characteristics:
Yttrium is a soft, silvery metal. Yttrium usually exists as a trivalent ion, Y3+, in its compounds. Most of its compounds are colorless.
Yttrium’s properties are very similar to those of the rare earth elements of the lanthanide series. Accordingly, yttrium is classified as one of the rare earth elements.
It is relatively stable in air as a result of an oxide film which forms on its surface.
The finely divided metal ignites in air when heated.
Yttrium reacts with water to form yttrium hydroxide plus hydrogen gas.
Interestingly, samples of rock and dust brought back from the Apollo moon landings show a high yttrium content. The yttrium content in lunar soil samples ranged from 54 to 213 parts per million. This compares with an average abundance of 33 parts per million in the earth’s crust. (6)
Yttrium has an exceptionally high affinity for oxygen, with a free energy of formation for the oxide of 1817 kJ mol-1, probably the greatest of any element. Yttrium also dissolves oxygen gas in relatively high concentrations. (7), (8)
Uses of Yttrium
Yttrium is often used in alloys, increasing the strength of aluminum and magnesium alloys.
It is also used as a deoxidizer for non-ferrous metals such as vanadium.
Yttrium is used as a catalyst in ethylene polymerization.
Yttrium-90, a radioactive isotope, is used in treatments for various cancers and is used in precision medical needles to sever pain-transmitting nerves in the spinal cord.
Yttrium oxide is the most important compound of yttrium. It is used to make the high-temperature superconductor YBCO (yttrium barium copper oxide). This substance becomes superconducting at -178 oC (meaning that it can be kept in a superconducting state using liquid nitrogen, rather than more expensive and more difficult to handle liquid helium).
Yttrium oxide is also used to make yttrium iron garnets (Y3 Fe5O12) which are very effective microwave filters, blocking some microwave frequencies, while allowing others through in communication devices such as satellites.
Yttrium doped with europium is used to produce phosphors, which provide the red color in color television tubes.
Abundance and Isotopes
Abundance earth’s crust: 33 parts per million by weight, 7.6 parts per million by moles
Abundance solar system: 10 parts per billion by weight, 0.1 parts per billion by moles
Cost, pure: $430 per 100g
Cost, bulk: $ per 100g
Source: Yttrium occurs in uranium ores and is present in nearly all the ‘rare earth’ minerals. It is recovered commercially by counter-current liquid-liquid extraction processes from monazite sand and bastnaesite. The metal can be isolated by reduction of the fluoride with calcium metal.
Isotopes: Yttrium has 25 isotopes whose half-lives are known, with mass numbers 79 to 103. Naturally occurring yttrium consists of its one stable isotope, 89Y.

References
- Per Enghag, Encyclopedia of the elements: technical data, history, processing, applications., John Wiley and Sons, 2004, p433,434.
- James L. Marshall and Virginia R. Marshall, Yttrium and Johan Gadolin pdf download.
- Paul Caro, Rare earths., Editorial Complutense., 1998, p28-29
- Mary Elvira Weeks, Discovery of the Elements., Kessinger Publishing, 2003, p169.
- John D. Corbett, Frank Harold Spedding A Biographical Memoir., National Academy Press 2001, p22.
- Apollo-15-Preliminary-Science-Report p199.
- A lanthanide Lanthology part II., Molycorp, Inc. Mountain Pass, CA, U.S.A., p54. pdf download.
- T.H. Okabe, et al., Electrochemical deoxidation of yttrium-oxygen solid solutions., Journal of Alloys and Compounds., 1996, 237, p150-154. pdf download.
Cite this Page
For online linking, please copy and paste one of the following:
<a href="https://www.chemicool.com/elements/yttrium.html">Yttrium</a>
or
<a href="https://www.chemicool.com/elements/yttrium.html">Yttrium Element Facts</a>
To cite this page in an academic document, please use the following MLA compliant citation:
"Yttrium." Chemicool Periodic Table. Chemicool.com. 18 Oct. 2012. Web. <https://www.chemicool.com/elements/yttrium.html>.
Very helpful thank you so very much sooo.